

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Orientation of Addition
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
19-1-2022
7617
Orientation of Addition
The direction of addition of hydrogen bromide to propene clearly depends on which end of the double bond the bromine atom attacks. The important question is which of the two possible carbon radicals that may be formed is the more stable, the 1-bromo-2-propyl radical, 5, or the 2-bromo-1-propyl radical, 6:
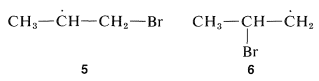
From C−H bond-dissociation energies of alkanes , the ease of formation and stabilities of the carbon radicals is seen to follow the sequence tertiary >> secondary >> primary. By analogy, the secondary 1-bromo-2-propyl radical, 5, is expected to be more stable and more easily formed than the primary 2-bromo-1-propyl radical, 66. The product of radical addition should be, and indeed is, 1-bromopropane:
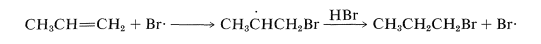
Other reagents, such as the halogens, also can add to alkenes and alkynes by both radical-chain and ionic mechanisms. Radical addition usually is initiated by light, whereas ionic addition is favored by low temperatures and no light. Nevertheless, it often is difficult to keep both mechanisms from operating at the same time. This is important even when the alkene is symmetrical because, although the adduct will then have the same structural formula regardless of mechanism, the stereochemical configurations may differ. Electrophilic addition of halogens generally is a stereospecific antarafacial addition, but radical-chain additions are less stereospecific.
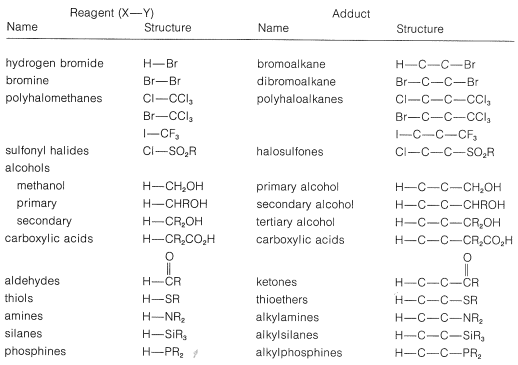
There are many reagents that add to alkenes only by radical-chain mechanisms. A number of these are listed in Table 10-3. They have in common a relatively weak bond, X−Y, that can be cleaved homolytically either by light or by chemical initiators such as peroxides. In the propagation steps, the radical that attacks the double bond does so to produce the more stable carbon radical. For addition to simple alkenes and alkynes, the more stable carbon radical is the one with the fewest hydrogens or the most alkyl groups at the radical center.
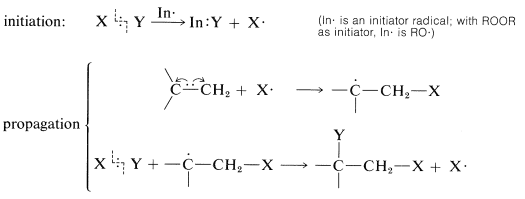
Table 10-3: Reagents that add to Alkenes by Radical-Chain Mechanisms
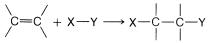
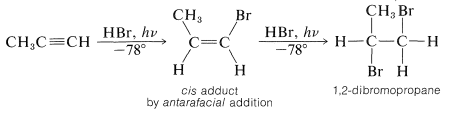
The principles of radical addition reactions of alkenes appear to apply equally to alkynes, although there are fewer documented examples of radical additions to triple bonds. Two molecules of hydrogen bromide can add to propyne first to give cis-1-bromopropene (by antarafacial addition) and then 1,2-dibromopropane:
Polymerization of Alkenes
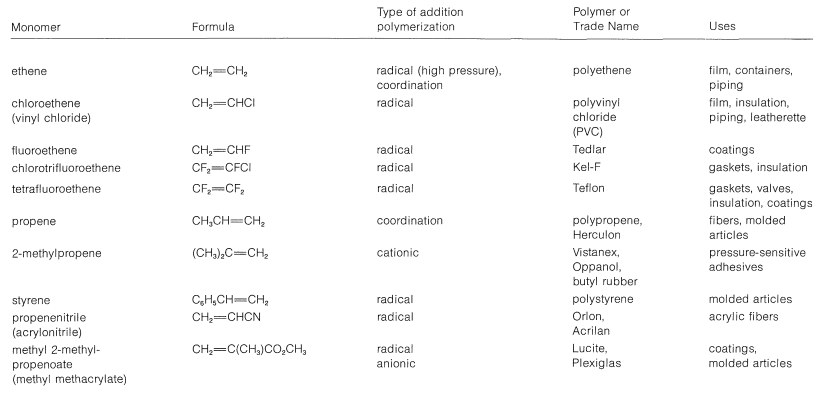
One of the most important technical reactions of alkenes is their conversion to higher-molecular-weight compounds or polymers (Table 10-4). A polymer is defined as a long-chain molecule with recurring structural units. Thus polymerization of propene gives a long-chain hydrocarbon with recurring 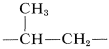 units:
units:
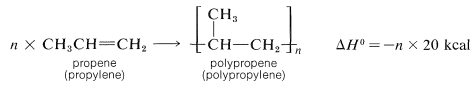
Table 10-4: Alkene Monomers and Their Polymers
Most technically important polymerizations of alkenes occur by chain mechanisms and may be classed as anion, cation, or radical reactions, depending upon the character of the chain-carrying species. In each case, the key steps involve successive additions to molecules of the alkene, the differences being in the number of electrons that are supplied by the attacking agent for formation of the new carbon-carbon bond. For simplicity, these steps will be illustrated by using ethene, even though it does not polymerize very easily by any of them:
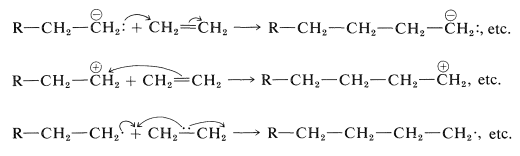
 الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















