

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Bonding considerations
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 390
15-2-2018
2999
Bonding considerations
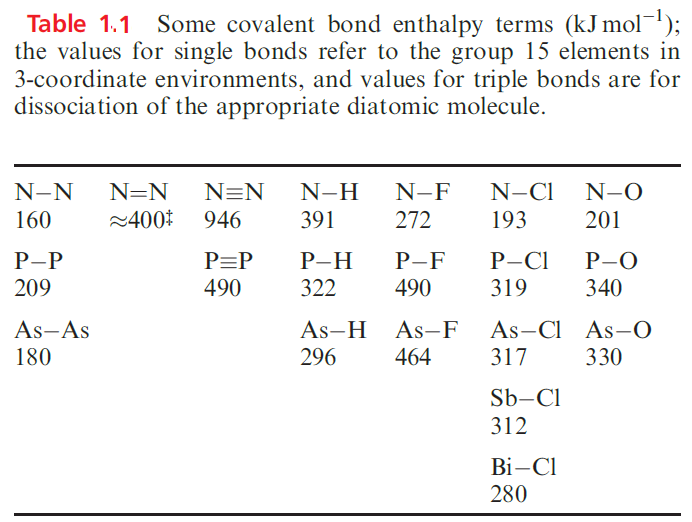
Analogies between groups 14 and 15 are seen if we consider certain bonding aspects. Table 1.1 lists some covalent bond enthalpy terms for group 15 elements. Data for most single bonds follow trends reminiscent of those in group 14
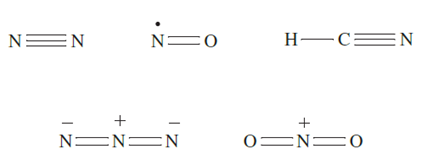
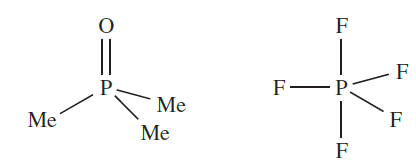
These observations, together with the absence of stable P-containing analogues of N2, NO, HCN, [N3]- and [NO2] indicate that strong (p–p)π-bonding is important only for the first member of group 15. It can be argued that differences between the chemistries of nitrogen and the heavier group 15 elements (e.g. existence of PF5, AsF5, SbF5 and BiF5, but not NF5) arise from the fact that an N atom is simply too small to accommodate five atoms around it. Historically, the differences have been attributed to the availability of d-orbitals on P, As, Sb and Bi, but not on N. However, even in the presence of electronegative atoms which would lower the energy of the d-orbitals, it is now considered that these orbitals play no significant role in hypervalent compounds of the group 15 (and later) elements. As we saw in Chapter 4, it is possible to account for the bonding in hypervalent molecules of the p-block elements in terms of a valence set of ns and np orbitals, and we should be cautious about using sp3d and sp3d2 hybridization schemes to describe trigonal bipyramidal and octahedral species of p-block elements. Although we shall show molecular structures of compounds in which P, As, Sb and Bi are in oxidation states of 5 (e.g. PCl5, [PO4]3-, [SbF6]-), the representation of a line between two atoms does not necessarily mean the presence of a localized twocentre two electron bond. Similarly, the representation of a double line between two atoms does not necessarily imply that the interaction comprises covalent σ- and π- contributions. For example, while it is often convenient to draw structures for Me3PO and PF5 as:
it is more realistic to show the role that charge-separated species play when one is discussing the electronic distribution in ions or molecules, i.e.
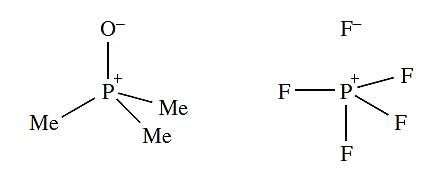
Furthermore, PF5 should really be represented by a series of resonance structures to provide a description that accounts for the equivalence of the two axial P_F bonds and the equivalence of the three equatorial P_F bonds. When we wish to focus on the structure of a molecule rather than on its bonding, charge-separated representations are not always the best option because they often obscure the observed geometry. This problem is readily seen by looking at the charge-separated representation of PF5, in which the trigonal bipyramidal structure of PF5 is not immediately apparent. The largest difference between groups 14 and 15 lies in the relative strengths of the N≡N (in N2) and N_N (in N2H4) bonds compared with those of C≡C and C_C bonds (Tables 1.1).
There is some uncertainty about a value for the N=N bond enthalpy term because of difficulty in choosing a reference compound, but the approximate value given in Table 1.1 is seen to be more than twice that of the N_N bond, whereas the C=C bond is significantly less than twice as strong as the C_C bond. While N2 is thermodynamically stable with respect to oligomerization to species containing N_N bonds, HC≡CH is thermodynamically unstable with respect to species with C_C bonds. Similarly, the dimerization of P2 to tetrahedral P4 is thermodynamically favourable. The σ- and π-contributions that contribute to the very high strength of the N≡N bond (which makes many nitrogen compounds endothermic and most of the others only slightly exothermic).
However, the particular weakness of the N_N single bond calls for comment. The O_O (146 kJ mol-1 in H2O2) and F_F (159 kJ mol-1 in F2) bonds are also very weak, much weaker than S_S or Cl_Cl bonds. In N2H4, H2O2 and F2, the N, O or F atoms carry lone pairs, and it is believed that the N_N, O_O and F_F bonds are weakened by repulsion between lone pairs on adjacent atoms (Figure 1.2).
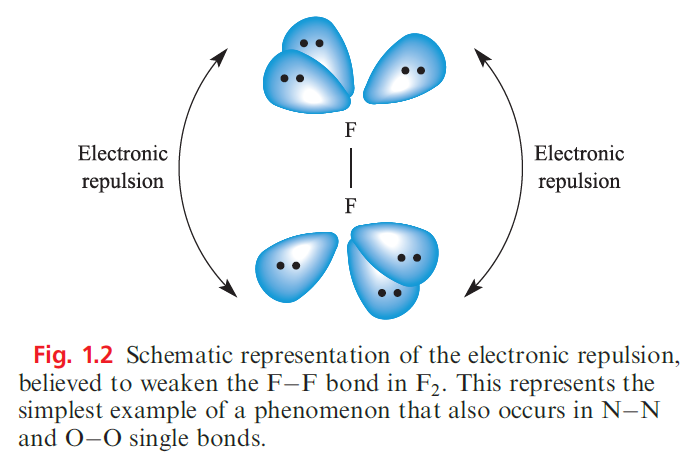
Lone pairs on larger atoms (e.g. in Cl2) are further apart and experience less mutual repulsion. Each N atom in N2 also has a nonbonding lone pair, but they are directed away from each other. Table 1.1 illustrates that N_O, N_F and N_Cl are also rather weak and, again, interactions between lone pairs of electrons can be used to rationalize these data. However, when N is singly bonded to an atom with no lone pairs (e.g. H), the bond is strong. In pursuing such arguments, we must remember that in a heteronuclear bond, extra energy contributions may be attributed to partial ionic character (see Section 1.15). Another important difference between N and the later group 15 elements is the ability of N to take part in strong hydrogen bonding (see Sections 9.6 and 14.5). This arises from the much higher electronegativity of N (xP = 3.0) compared with values for the later elements (xP values: P, 2.2; As, 2.2; Sb, 2.1; Bi, 2.0). The ability of the first row element to participate in hydrogen bonding is also seen in group 16 (e.g. O_H….O and N_H…..O interactions) and group 17 (e.g. O_H…..F, N_H…..F interactions). For carbon, the first member of group 14, weak hydrogen bonds (e.g. C_H……O interactions) are important in the solid state structures of small molecules and biological systems.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















