

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Uses of The group 15 elements
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 387
12-2-2018
2531
Uses of The group 15 elements
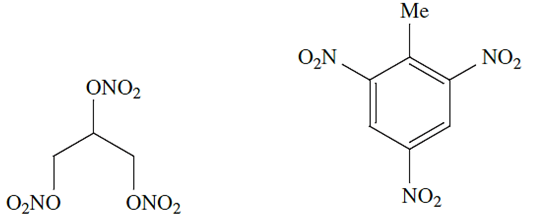
In the US, N2 ranks second in industrial chemicals, and a large proportion of N2 is converted to NH3 . Gaseous N2 is widely used to provide inert atmospheres, both industrially (e.g. in the electronics industry during the production of transistors etc.) and in laboratories. Liquid N2 (bp 77 K) is an important coolant (Table 1.1) with applications in some freezing processes.
Nitrogen-based chemicals are extremely important, and include nitrogenous fertilizers nitric acid and nitrate salts, explosives such as nitroglycerine and trinitrotoluene nitrite salts (e.g. in the curing of meat where they prevent discoloration by inhibiting oxidation of blood), cyanides and azides.
By far the most important application of phosphorus is in phosphate fertilizers we highlight this use and possible associated environmental problems. Bone ash (calcium phosphate) is used in the manufacture of bone china. Most white phosphorus is converted to H3PO4, or to compounds such as P4O10, P4S10, PCl3 and POCl3. Phosphoric acid is industrially very important and is used on a large scale in the production of fertilizers, detergents and food additives. It is responsible for the sharp taste of many soft drinks, and is used to remove oxide and scale from the surfaces of iron and steel. Phosphorus trichloride is also manufactured on a large scale; it is a precursor to many organophosphorus compounds, including nerve gases flame retardants and insecticides. Phosphorus is important in steel manufacture and phosphor bronzes. Red phosphorus is used in safety matches and in the generation of smoke (e.g. fireworks, smoke bombs).
Arsenic salts and arsines are extremely toxic, and uses of arsenic compounds in weedkillers, sheep- and cattle -dips, and poisons against vermin are less widespread than was once the case. Antimony compounds are less toxic, but large doses result in liver damage. Potassium antimony tartrate (tartar emetic) was used medicinally as an emetic and expectorant but has now been replaced by less toxic reagents. Bismuth is one of the less toxic heavy metals and compounds, such as the subcarbonate (BiO)2CO3, find use in stomach remedies including treatments for peptic ulcers. Arsenic is a doping agent in semiconductors and GaAs has widespread uses in solid state devices and semiconductors. Uses of As include those in the semiconductor industry, in alloys (e.g. it increases the strength of Pb) and in batteries. Sb2O3 is used in paints, adhesives and plastics, and as a flame retardant . Uses of Sb2S3 include those in photoelectric devices and electrophotographic recording materials, and as a flame retardant. Major uses of bismuth are in alloys (e.g. with Sn) and as Bi-containing compounds such as BiOCl in cosmetic products (e.g. creams, hair dyes and tints). Other uses are as oxidation catalysts and in high-temperature superconductors; Bi2O3 has many uses in the glass and ceramics industry, and for catalysts and magnets. The move towards lead-free solders has resulted in increased use of Bi-containing solders, e.g. Sn/Bi/Ag alloys. A number of other applications are emerging in which Bi substitutes for Pb, for example in bismuth shot for game-hunting.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















