
Amino Acids Share Common Structural Features
 المؤلف:
David L. Nelson, Michael M. Cox
المؤلف:
David L. Nelson, Michael M. Cox
 المصدر:
Book or Source : Lehninger Principles of Biochemistry 6th ed 2012
المصدر:
Book or Source : Lehninger Principles of Biochemistry 6th ed 2012
 الجزء والصفحة:
p 76
الجزء والصفحة:
p 76
 9-4-2017
9-4-2017
 6299
6299
Amino Acids Share Common Structural Features
All 20 of the common amino acids are α-amino acids. They have a carboxyl group and an amino group bonded to the same carbon atom (the α carbon) (Fig. 1.1). They differ from each other in their side chains, or R groups, which vary in structure, size, and electric charge, and which influence the solubility of the amino acids in water. In addition to these 20 amino acids there are many less common ones. Some are residues modified after a protein has been synthesized; others are amino acids present in living organisms but not as constituents of proteins. The common amino acids of proteins have been assigned three-letter abbreviations and one-letter
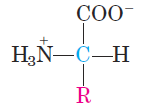
FIGURE 1.1 General structure of an amino acid. This structure is common to all but one of the α-amino acids. (Proline, a cyclic amino acid, is the exception.) The R group or side chain (red) attached to the α carbon (blue) is different in each amino acid.
symbols which are used as shorthand to indicate the composition and sequence of amino acids polymerized in proteins. Two conventions are used to identify the carbons in an amino acid—a practice that can be confusing. The additional carbons in an R group are commonly designated β, γ, δ ,ε and so forth, proceeding out from the α carbon. For most other organic molecules, carbon atoms are simply numbered from one end, giving highest priority (C-1) to the carbon with the substituent containing the atom of highest atomic number. Within this latter convention, the carboxyl carbon of an amino acid would be C-1 and the α carbon would be C-2. In some cases, such as amino acids with heterocyclic R groups, the Greek lettering system is ambiguous and the numbering convention is therefore used.
For all the common amino acids except glycine, the α carbon is bonded to four different groups: a carboxyl group, an amino group, an R group, and a hydrogen atom (Fig. 1.1; in glycine, the R group is another hydrogen atom). The α-carbon atom is thus a chiral center . Because of the tetrahedral arrangement of the bonding orbitals around the α-carbon atom, the four different groups can occupy two unique spatial arrangements, and thus amino acids have two possible stereoisomers. Since they are nonsuperimposable mirror images of each other (Fig. 1.2), the two forms represent a class of stereoisomers called enantiomers. All molecules with a chiral center are also optically active—that is, they rotate plane-polarized light. Special nomenclature has been developed to specify the absolute configuration of the four substituents of asymmetric carbon atoms. The absolute configurations of simple sugars and amino acids are specified by the D, L system (Fig. 1.3), based on the absolute configuration of the three-carbon sugar glyceraldehyde, a convention proposed by Emil Fischer in 1891. (Fischer knew what groups surrounded the asymmetric carbon of glyceraldehyde but had to guess at their absolute configuration; his guess was later confirmed by x-ray diffraction analysis.) For all chiral compounds, stereoisomers having a configuration related to that of L-glyceraldehyde are designated L, and stereoisomers related to D-glyceraldehyde are designated D. The functional groups of L-alanine are matched with those of Lglyceraldehyde by aligning those that can be interconverted by simple, one-step chemical reactions. Thus the carboxyl group of L-alanine occupies the same position about the chiral carbon as does the aldehyde group of L-glyceraldehyde, because an aldehyde is readily converted to a carboxyl group via a one-step oxidation. Historically, the similar l and d designations were used for levorotatory (rotating light to the left) and dextrorotatory (rotating light to the right). However, not all
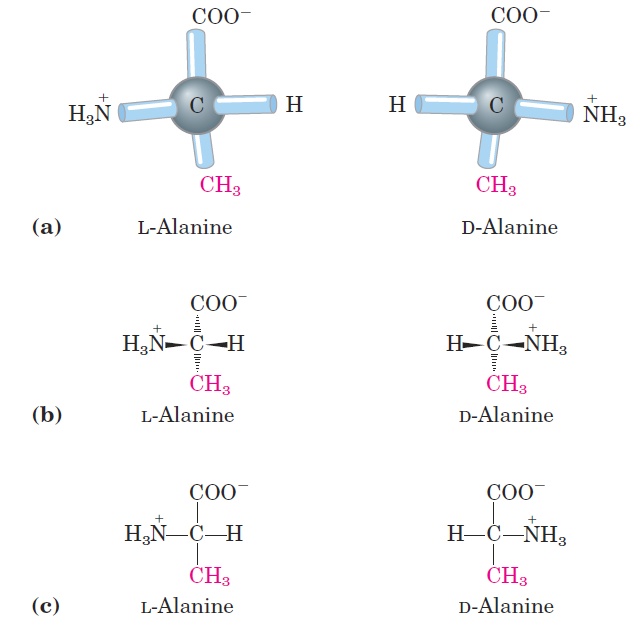
FIGURE 1.2 Stereoisomerism in α-amino acids. (a)The two stereoisomers of alanine, L- and D-alanine, are nonsuperimposable mirror images of each other (enantiomers). (b, c) Two different conventions for showing the configurations in space of stereoisomers. In perspective formulas (b) the solid wedge-shaped bonds project out of the plane of the paper, the dashed bonds behind it. In projection formulas (c) the horizontal bonds are assumed to project out of the plane of the paper, the vertical bonds behind. However, projection formulas are often used casually and are not always intended to portray a specific stereochemical configuration.
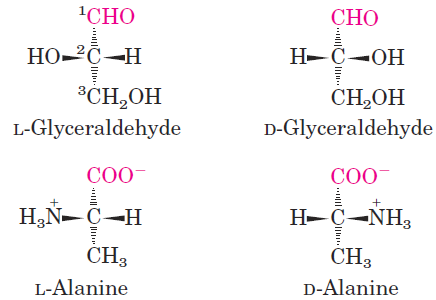
FIGURE 1.3 Steric relationship of the stereoisomers of alanine to the absolute configuration of L- and D-glyceraldehyde. In these perspective formulas, the carbons are lined up vertically, with the chiral atom in the center. The carbons in these molecules are numbered beginning with the terminal aldehyde or carboxyl carbon (red), 1 to 3 from top to bottom as shown. When presented in this way, the R group of the amino acid (in this case the methyl group of alanine) is always below the α carbon. L-Amino acids are those with the α-amino group on the left, and D-amino acids have the α-amino group on the right.
L-amino acids are levorotatory, and the convention shown in Figure 1.3 was needed to avoid potential ambiguities about absolute configuration. By Fischer’s convention, L and D refer only to the absolute configuration of the four substituents around the chiral carbon, not to optical properties of the molecule. Another system of specifying configuration around a chiral center is the RS system, which is used in the systematic nomenclature of organic chemistry and describes more precisely the configuration of molecules with more than one chiral center (see p. 18).
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


