

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Silicides
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 358
5-4-2017
2085
Silicides
The structures of the metal silicides (prepared by direct combination of the elements at high temperatures) are diverse, and a full discussion of the structures is beyond the scope of this book.† Some examples of their solid state structural types are:
- isolated Si atoms (e.g. Mg2Si, Ca2Si);
-
Si2-units (e.g. U3Si2);
-
Si4-units (e.g. NaSi, KSi, CsSi)
- Sin-chains (e.g. CaSi);
- planar or puckered hexagonal networks of Si atoms (e.g. b-USi2, CaSi2);
- three-dimensional network of Si atoms (e.g. SrSi2, a-USi2).
The Si4-units present in the alkali metal silicides are noteworthy. The [Si4]4- anion is isoelectronic with P4 and the solid state structures of several group 1 metal silicides contain tetrahedral Si4-units, but these are not isolated anions. The structure of Cs4Si4 comes close to featuring discrete, tetrahedral [Si4]4- ions, but significant cation–anion interactions exist. The silicide K3LiSi4 possesses tetrahedral Si4-units linked by Li ions to give infinite chains, and in K7LiSi8, pairs of Si4-units are connected as shown in structure 1.1 with additional interactions involving K ions.
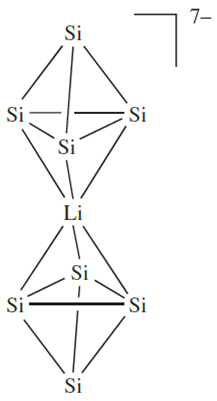
(1.1)
Silicides are hard materials, but their melting points are generally lower than those of the metal carbides. Treatment of Mg2Si with dilute acids gives mixtures of silanes. The properties of some silicides make them useful as refractory materials (e.g. Fe3Si and CrSi2); Fe3Si is used in magnetic tapes and disks to increase their thermal stability.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















