

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Polar covalent bonding: Creating partial charges
المؤلف:
John T. Moore, EdD
المصدر:
Chemistry Essentials For Dummies
الجزء والصفحة:
p 84
5-1-2017
3043
Polar covalent bonding: Creating partial charges
If the two atoms involved in the covalent bond are not the same, the bonding pair of electrons is pulled toward one atom, with that atom taking on a slight (partial) negative charge and the other atom taking on a partial positive charge.
In most cases, the molecule has a positive end and a negative charge. end, called a dipole (think of a magnet). Figure 1-1 shows a couple of examples of molecules in which dipoles have formed. (The δ symbol by the charges is the lowercase Greek letter delta, and it refers to a partial charge.)
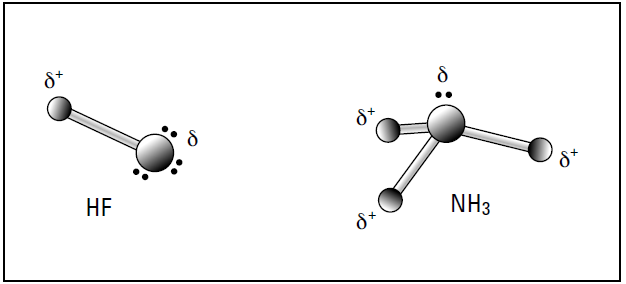
Figure 1-1: Polar covalent bonding in HF and NH3
In hydrogen fluoride (HF), the bonding electron pair is pulled much closer to the fluorine atom than to the hydrogen atom, so the fluorine end becomes partially negatively charged and the hydrogen end becomes partially positively charged. The same thing takes place in ammonia (NH3): The nitrogen has a greater electronegativity than hydrogen, so the bonding pairs of electrons are more attracted to it than to the hydrogen atoms. The nitrogen atom takes on a partial negative charge, and each hydrogen atom takes on a partial positive charge.
The presence of a polar covalent bond explains why some substances act the way they do in a chemical reaction: Because a polar molecule has a positive end and a negative end, it can attract the part of another molecule that has the opposite charge. In addition, a polar covalent molecule can act as a weak electrolyte because a polar covalent bond allows the substance to act as a conductor. So if a chemist wants a material to act as a good insulator (a device used to separate conductors), he or she looks for a material with as weak of a polar covalent bond as possible.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















