
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
MULTIPLE ELECTRONS: THE MERCURY SPECTRUM
المؤلف:
Mark Csele
المصدر:
FUNDAMENTALS OF LIGHT SOURCES AND LASERS
الجزء والصفحة:
p71
9-3-2016
2091
MULTIPLE ELECTRONS: THE MERCURY SPECTRUM
Unlike sodium, mercury has two valence electrons. These two electrons may have their spins in opposite directions, in which case they effectively cancel each other out, resulting in a spin of s = 0. The subscript j, then, is simply the orbital angular momentum l. If the spins are in the same direction, the contribution from spin is s = 1. Consider the 6S state. l = 0, but spin can contribute a value of 0 or 1, so the 3S state becomes two states: 6S0 and 6S1. The 6P state, with l = 0 or 1, can now yield values for j of 0, 1, and 2. These levels are designated as 63P0, 63P1 , and 63P2 , where the superscript 3 denotes that the multiplicity of the level is three.
Where two electrons are considered, there are two basic types of spin states, triplets and singlets. A triplet results from a group of three symmetric states for spin in which the resulting total spin is -1, 0, or +1, yielding j values of 0, 1, and 2, as stated above. A singlet results when the spin of an excited electron is antiparallel to that of the remainder of the atom or molecule: an asymmetric state where the total combined spin is 0. Consider the simplest two-electron example in helium with two electrons: If the excited electron has a spin in the direction opposite to that of the remaining ground-state electron (which stays in a 1s state), a singlet results (with the excited species called parahelium); if the spin of the excited electron is in the same direction parallel to that of the remaining ground-state electron, a triplet results (with the excited species called orthohelium).
Transitions resulting from this splitting of energy levels in an atom such as mercury show a triplet of three spectral lines. Unlike the closely spaced lines of the doublet in the previous example, these lines will be separated by a much larger spacing, since the energy difference between these levels is much larger. In the case of the 6P2 level, both electrons are spinning in the same direction as that of angular orbital momentum, so the energy is the lowest in this case. Another feature of multielectron atoms is the fact that no two electrons can have exactly the
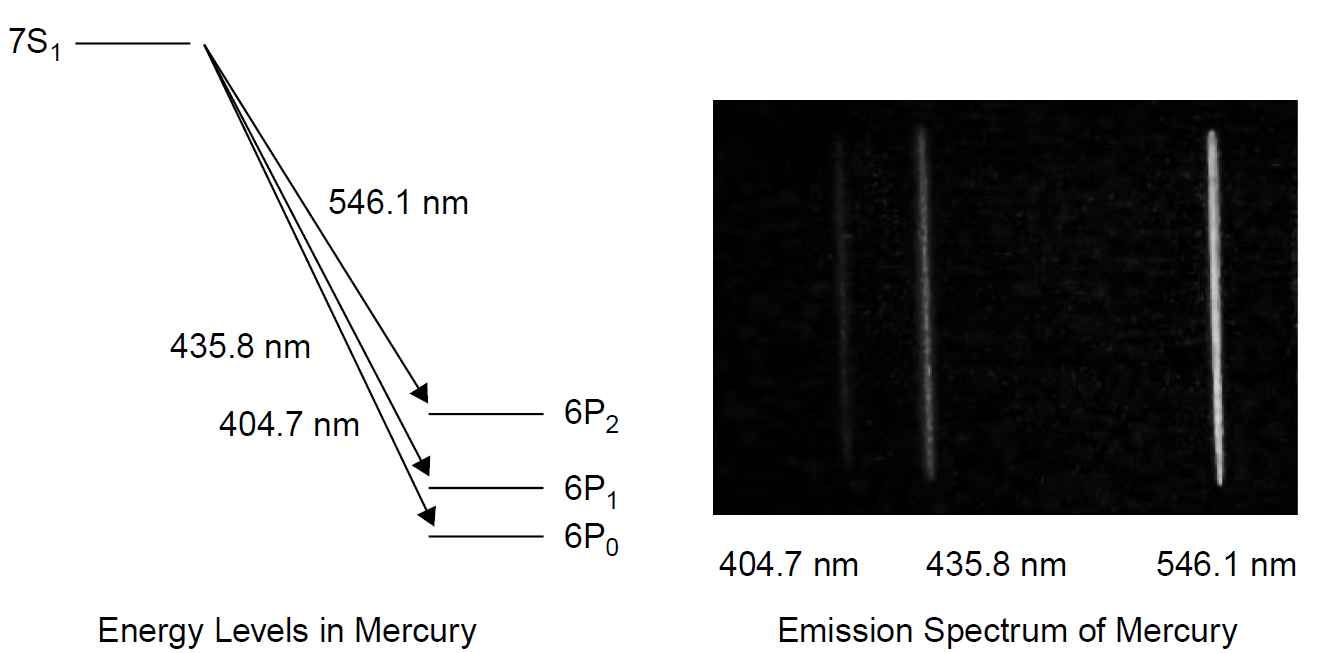
Figure 1.1. Mercury energy levels and the fine structure.
same quantum numbers. This is, of course, the Pauli exclusion principle and guides us in understanding why an S shell can hold only two electrons and a P shell only six electrons.
 الاكثر قراءة في مواضيع عامة في الليزر
الاكثر قراءة في مواضيع عامة في الليزر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















