

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Heterochromatin Formation Requires MicroRNAs
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
18-6-2021
3246
Heterochromatin Formation Requires MicroRNAs
Key concept
- MicroRNAs can promote heterochromatin formation.
As described in the chapters titled Epigenetics I and Epigenetics II, heterochromatin is one of the major subdivisions that can be seen in chromosomes. It is visually different when stained because it is more condensed than euchromatin. It is late replicating and has few genes. The underlying DNA sequence is different from euchromatin in that it consists primarily of simple sequence satellite DNA organized in giant tandem blocks. Small islands of genes containing unique sequences of DNA are found within heterochromatin. These simple sequence regions were once thought to be largely transcriptionally silent, but it is now known that virtually the entire genome is transcribed, including the simple sequence satellite DNA that is often found surrounding centromeres and the repeats found in telomeres. In fact, transcripts from these sequences are used to organize the heterochromatin structure and repress its transcription.
The centromeric heterochromatin of the fission yeast Schizosaccharomyces pombe has been a model for understanding heterochromatin formation. The outer region repeat sequences of the heterochromatin are transcribed into ncRNAs by RNA polymerase II. This transcript is copied by an RNA-dependent RNA polymerase (RDRP) to give a double-stranded RNA, which is processed into siRNAs. Plants use a variation of the RNA polymerase, RNA polymerase IVb/V, to amplify the ncRNA signal.
In Drosophila, the siRNAs have been linked to sister chromatid recognition within X chromosomes, to distinguish X chromosomes from the autosomes and for dosage compensation between males and females.
In a manner similar to that described earlier in the section How Does RNA Interference Work?, the RNA is processed by Dicer. An alternative processing pathway through the TRAMP (Trf4-Air1-Mtr4 polyadenylation) exosome complex also exists. The complex to which the fragments are delivered is called RNA-induced transcriptional silencing (RITS). RITS contains an Argonaute subunit, Ago1. RITS and RDRP are in a complex together. Again, as shown earlier, RITS uses the siRNA as a targeting mechanism back to its origin to begin the process of repressing transcription.
This entails the recruitment of factors to begin chromatin modification, such as a histone H3K9 methyltransferase , as seen in FIGURE 1. If this methyltransferase is tethered to euchromatin, heterochromatin will be induced at that site. The only function for the outer repeats and the siRNA is to recruit the methyltransferase. An analogous system is found in Drosophila, as described earlier, for rasiRNAs that are targeted to the alternate RISC complex containing Piwi, Aubergine, and Ago3 proteins.
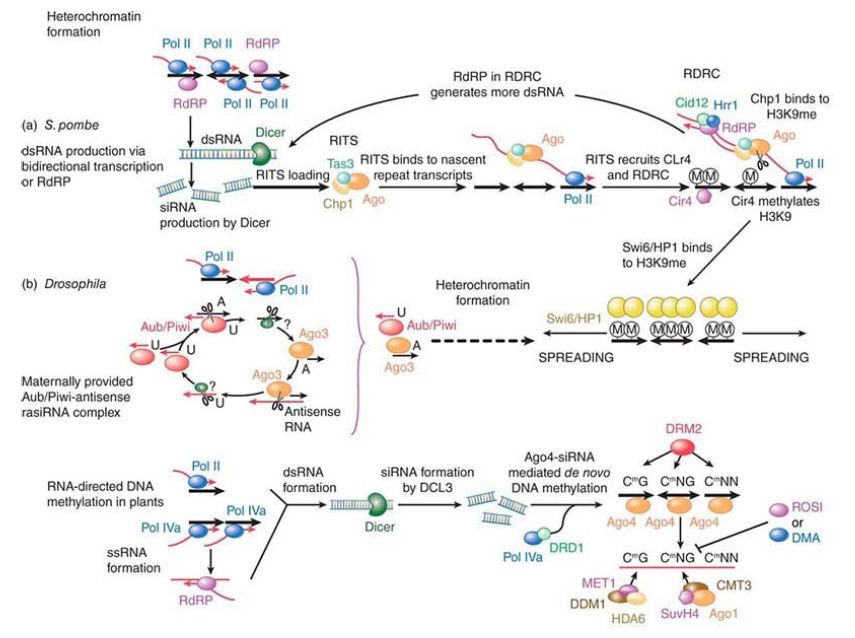
FIGURE 1.(a) Heterochromatin formation in Schizosaccharomyces pombe. DNA repeats produce doublestranded (ds)RNAs through bidirectional transcription or RNAdependent RNA synthesis. dsRNAs are cut into small interfering (si)RNAs that are loaded into an RNA-induced transcriptional silencing complex (RITS) that consists of Ago; Tas3, an S. pombe–specific protein; and Chp1, a chromodomain-containing protein. RITS finds the DNA repeats through siRNA base pairing with the nascent transcript and recruits the RNA-directed RNA polymerase complex (RDRC) and Clr4, a histone methyltransferase that methylates histone H3 at lysine 9 (H3K9me). RdRP in RDRC uses the Ago-cut nascent RNA as a template to synthesize more dsRNA, which, in turn, will be cut into siRNAs to reinforce heterochromatin formation. Chp1 in the RITS complex binds to H3K9me, resulting in stable interaction of RITS and heterochromatic DNA. H3K9me also binds to another chromodomain protein, Swi6 (an HP1 homolog), leading to the spreading of heterochromatin. (b) Heterochromatin formation in Drosophila. Repeat-associated small interfering RNAs (rasiRNAs) are produced in a Dicer-independent, Aub/Piwi–Ago3 “ping-pong” mechanism. Aub/Piwi associates with antisense rasiRNAs with a preference for a U at the 5′ end, whereas Ago associates with sense-strand derived rasiRNA with a preference to an A at nucleotide 10. Aub/Piwi–rasiRNA complex binds to sensestrand RNA via a 10-nucleotide complementary sequence. Aub/Piwi cleaves sense-strand RNA, producing sense rasiRNA precursor. A yet-to-be-identified nuclease (denoted “?”) generates the sense rasiRNAs that associate with Ago3. In turn, Ago3-sense siRNA binds to antisense RNA and generates more antisense rasiRNAs. In this ping-pong model, the initial Aub/Piwi–rasiRNA complex is maternally deposited. The resulting rasiRNA complexes initiate heterochromatin formation (dotted arrow line). As in yeast, H3K9me binds to an HP1 protein, leading to the spreading of heterochromatin. A similar mechanism has been reported in mammals.
Reprinted from Cell, vol. 130, Y. Bei, S. Pressman, and R. Carthew, SnapShot: Small RNAMediated …, pp. 756.e1–756.e2. Copyright 2007, with permission from Elsevier [http://www.sciencedirect.com/science/journal/00928674].
Telomere heterochromatin is also transcribed. Similar to centromeric heterochromatin, telomeres are also composed of repeat-sequence DNA. These are transcribed into large ncRNAs called telomere repeat-containing RNA, or TERRA. The G-rich TERRA folds into G quadruplex structures, as shown in FIGURE 2. A number of proteins bind to TERRA and are involved in the control of telomerase-directed replication at the telomere .

FIGURE 2.G-quartet and G-quadruplex structures and topologies. The guanine bases are connected by Hoogsteen hydrogen–bonded base pairing. A central monovalention is necessary for formation and stabilization. Reprinted from Yan Xu, et al. Proc. Natl. Acad. Sci. USA 107 (2010): 14579–14584. Copyright © 2010 National Academy of Sciences, U.S.A.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















