


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-10-2020
Date: 14-9-2018
Date: 17-7-2019
|
In terms of reaction conditions and the factors affecting the rates of the reaction, there is no difference whatsoever between these alkenes and the symmetrical ones described above. The problem comes with the orientation of the addition - in other words, which way around the hydrogen and the halogen add across the double bond.
Orientation of addition
If HCl adds to an unsymmetrical alkene like propene, there are two possible ways it could add. However, in practice, there is only one major product.
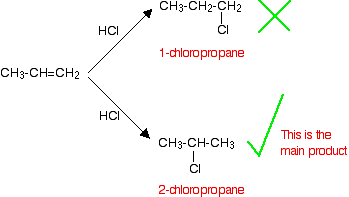
This is in line with Markovnikov's Rule which says:
In this case, the hydrogen becomes attached to the CH2 group, because the CH2 group has more hydrogens than the CH group. Notice that only the hydrogens directly attached to the carbon atoms at either end of the double bond count. The ones in the CH3 group are totally irrelevant.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|