
Phosphorus halides
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
Inorganic Chemistry
المصدر:
Inorganic Chemistry
 الجزء والصفحة:
p 406
الجزء والصفحة:
p 406
 23-2-2018
23-2-2018
 2966
2966
Phosphorus halides
Phosphorus forms the halides PX3 (X = F, Cl, Br and I) and PX5 (X= F, Cl and Br); PI5 is unknown. Most are made by direct combination of the elements with the product determined by which element is in excess; PF3, however, must be made by reaction below and a convenient synthesis of PF5 is from KPF6 (see below). The halides are all hydrolysed by water although PF3 reacts only slowly.
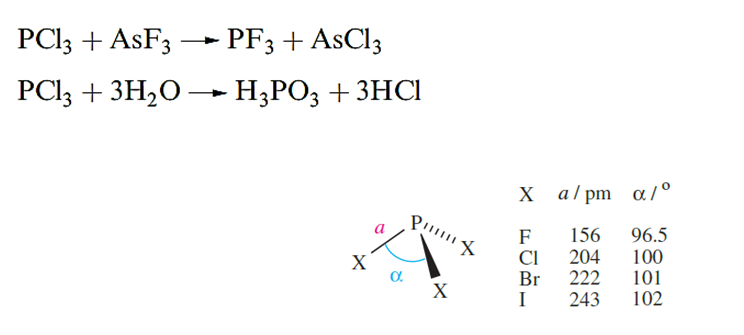
Each of the trihalides has a trigonal pyramidal structure above. Phosphorus trifluoride is a very poisonous, colourless and odourless gas. It has the ability (like CO) to form complexes with metals and Lewis acids such as BH3, and its toxicity arises from complex formation with haemoglobin. Protonation of PF3 can be achieved when HF/SbF5 is used as the acid although an analogous reaction does not occur with AsF3. [HPF3][SbF6]:HF is thermally unstable, but low-temperature structural data show that the tetrahedral [HPF3] ion has bond lengths of P_H = 122 and P_F =149 pm.

The reaction of PF3 with Me4NF in MeCN gives [Me4N][PF4]. The [PF4]- ions are disphenoidal in shape, consistent with VSEPR theory, i.e. the structure is derived from a trigonal bipyramid with a lone pair of electrons occupying one equatorial position. In solution, [PF4]- is stereochemically non-rigid and the mechanism of F atom exchange is probably by Berry pseudo-rotation. When treated with an equimolar amount of water, [PF4]- hydrolyses according to equation below. With an excess of water, [HPF5]- also hydrolyses to [HPO2F]- making this the only product of the overall hydrolysis of [PF4] -.
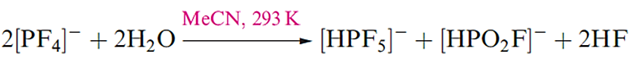
Phosphorus trichloride is a colourless liquid (mp 179.5 K, bp 349 K) which fumes in moist air and is toxic. Its reactions include those in scheme below POCl3 is an important reagent for the preparation of phosphate esters.

Single-crystal X-ray diffraction (at 109 K) data show that PF5 has a trigonal bipyramidal structure, 14.32. In solution, the molecule is fluxional on the NMR spectroscopic timescale and a doublet is observed in the 19FNMRspectrum, i.e. all 19F environments are equivalent and couple with the 31P nucleus. This stereochemical non-rigidity is another example of Berry pseudo-rotation. Electron diffraction data show that in the gas phase, PCl5 has a molecular, trigonal bipyramidal structure (P_Clax = 214, P_Cleq = 202 pm), provided that thermal dissociation into PCl3 and Cl2 is prevented by the presence of an excess of Cl2. In the solid state, however, tetrahedral [PCl4] (P_Cl = 197 pm) and octahedral [PCl6]-(P_Cl = 208 pm) ions are present, and the compound crystallizes with a CsCl lattice (Figure 5.16). In contrast, PBr5 (which dissociates in the gas phase to PBr3and Br2) crystallizes in the form of [PBr4]+Br-. The mixed halide PF3Cl2 is of particular interest. It is obtained as a gas (bp 280 K) from the reaction of PF3 and Cl2 and has a molecular structure with equatorial Cl atoms. However, when PCl5 reacts with AsF3 in AsCl3 solution, the solid product [PCl4][PF6]- (mp ≈ 403K) is isolated. Solid PI5 has not been isolated, but the isolation of the salts [PI4][AsF6]- (from the reaction of PI3 and [I3][AsF6-) and [PI4][AlCl4]- (from the reaction between PI3, ICl and AlCl3) confirms the existence of the tetrahedral tetraiodophosphonium ion. The reaction of PBr3 with [I3][AsF6] leads to a mixture of [PBr4][AsF6], [PBr3I][AsF6] and small amounts of [PBr2I2][AsF6]. Selective formation of [PBr4][AsF6] can be achieved by treating PBr3 with [Br3]+[AsF6]-.
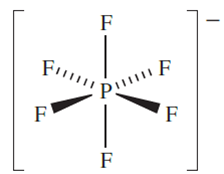
Phosphorus pentafluoride is a strong Lewis acid and forms stable complexes with amines and ethers. The hexafluorophosphate ion, [PF6]- is made in aqueous solution by reacting H3PO4 with concentrated HF; [PF6]- is isoelectronic and isostructural with [SiF6]2-. Salts such as [NH4][PF6] are commercially available, and [PF6]- is used to precipitate salts containing large organic or complex cations. Solid KPF6 (prepared as in Figure 1.1) decomposes on heating to give PF5 and this route is a useful means of preparing PF5.
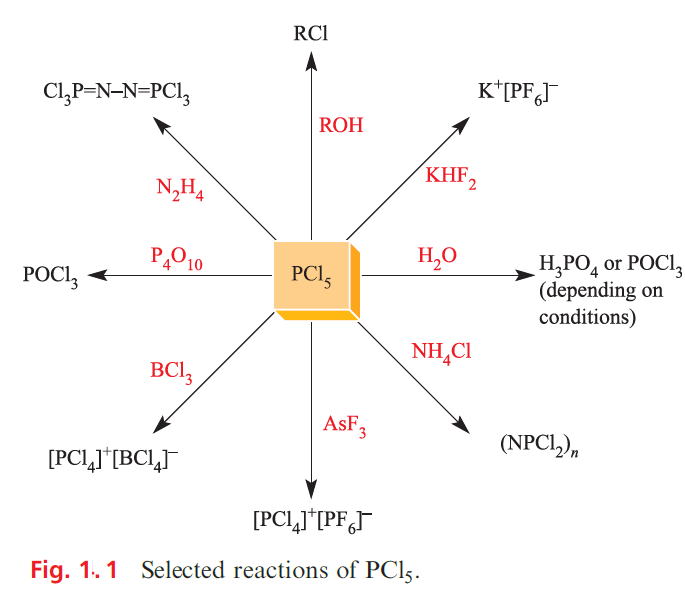
Phosphorus pentachloride is an important reagent, and is made industrially by the reaction of PCl3 and Cl2; selected reactions are given in Figure 1.1. Of the lower halides P2X4, the most important is the red, crystalline P2I4 (mp 398 K) which can be made by reacting white phosphorus with I2 in CS2. In the solid state, molecules of P2I4 adopt a trans (staggered) conformation . In many of its reactions, P2I4 undergoes P_P bond fission, e.g. dropping H2O on P2I4 in an inert atmosphere produces [PH4]I.
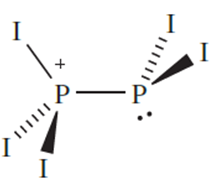
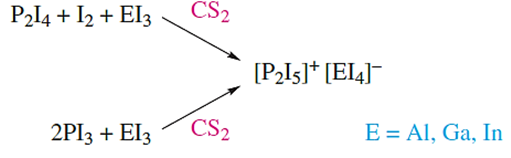
Salts of [P2I5]+ can be obtained according to scheme 14.76. In these salts, the [P2I5]+ ion exists only in the solid state; the 31P NMR spectra of CS2 solutions of dissolved samples show a singlet at≈178, consistent with the presence of PI3 rather than [P2I5]+. In contrast, solution 31P NMR spectra have been obtained for [P2I5]+ in the presence of the [Al{OC(CF3)3}4]- anion.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


