
Sulfides of The group 14 elements
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
Inorganic Chemistry
المصدر:
Inorganic Chemistry
 الجزء والصفحة:
p 377
الجزء والصفحة:
p 377
 9-2-2018
9-2-2018
 2490
2490
Sulfides of The group 14 elements
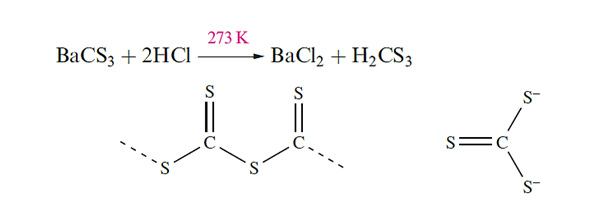
The disulfides of C, Si, Ge and Sn show the gradation in properties that might be expected to accompany the increasingly metallic character of the elements. Pertinent properties of these sulfides are given in Table 1.1. Lead(IV) is too powerful an oxidizing agent to coexist with S2- , and PbS2 is not known. Carbon disulfide is made by heating charcoal with sulfur at 1200K, or by passing CH4 and sulfur vapour over Al2O3 at 950 K. It is highly toxic (by inhalation and absorption through the skin) and extremely flammable, but is an excellent solvent which is used in the production of rayon and cellophane. Carbon disulfide is insoluble in water, but is, by a narrow margin, thermodynamically unstable with respect to hydrolysis to CO2 and H2S. However, this reaction has a high kinetic barrier and is very slow. Unlike CO2, CS2 polymerizes under high pressure to give a black solid. When shaken with solutions of group 1 metal sulfides, CS2 dissolves readily to give trithiocarbonates, M2CS3, which contain the [CS3]2- ion the sulfur analogue of [CO3]2-. Salts are readily isolated, e.g. Na2CS3 forms yellow needles (mp 353K). The free acid H2CS3 separates as an oil when salts are treated with hydrochloric acid and behaves as a weak acid in aqueous solution: pKa(1( = 2.68, pKa(2) = 8.18.
The action of an electric discharge on CS2 results in the formation of C3S2, a red liquid which decomposes at room temperature, producing ablack polymer (C3S2)x. When heated, C3S2 explodes. Incontrast to CO, CS is a short-lived radical species whichdecomposes at 113 K; it has, however, been observed in theupper atmosphere.
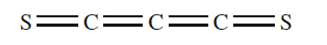
Several salts of the [C2S4]2- anion are known although the free acid (an analogue of oxalic acid) has not been isolated.
In [Et4N]2[C2S4], the anion has D2d symmetry, i.e. the dihedral angle between the planes containing the two CS2-units is 908 whereas in [Ph4P]2[C2S4].6H2O, this angle is 79.58. It is interesting to compare these structural data with those for salts of the related oxalate ion, [C2O4]2-. The solid state structures of anhydrous alkali metal oxalates respond to an increase in the size of the metal ion. In Li2C2O4, Na2C2O4, K2C2O4 and in one polymorph of Rb2C2O4, the [C2O4]2- ion is planar. In the second polymorph of Rb2C2O4 and in Cs2C2O4, the [C2O4]2- ion adopts a staggered conformation.
Oxalate salts in general tend to exhibit planar anions in the solid state. The C_C bond length (157 pm) is consistent with a single bond and indicates that the planar structure is not a consequence of π-delocalization but is, instead, a result of intermolecular interactions in the crystal lattice.
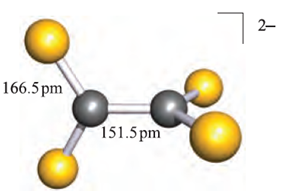
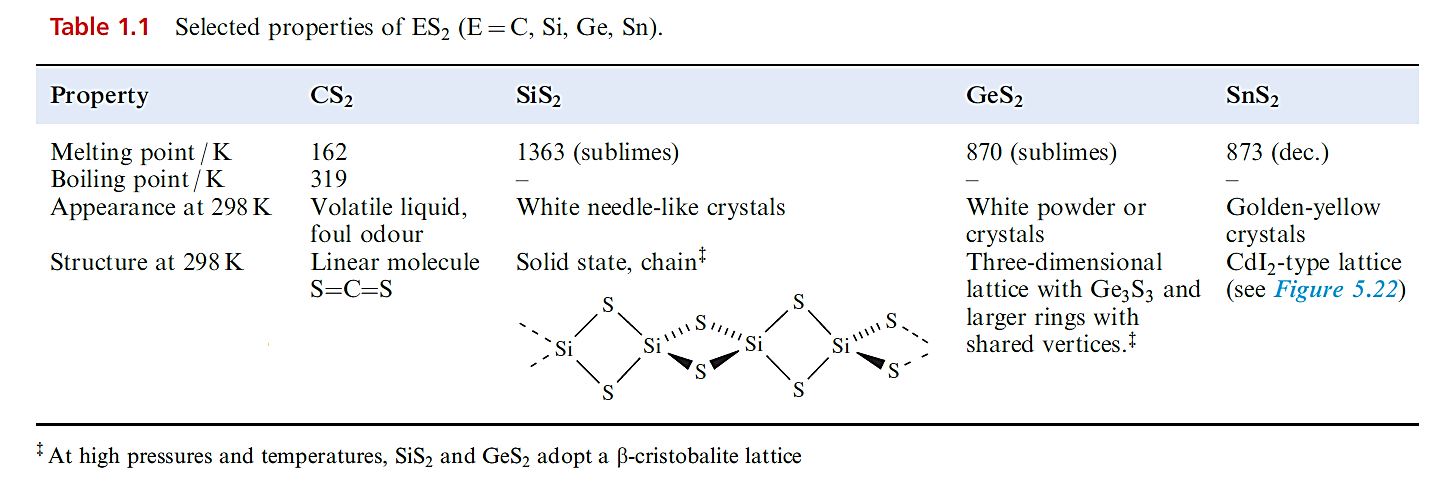
Silicon disulfide is prepared by heating Si in sulfur vapour. Both the structure of this compound (Table 1.1) and the chemistry of SiS2 show no parallels with SiO2; SiS2 is instantly hydrolysed.
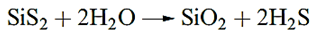
The disulfides of Ge and Sn (Table 1.1) are precipitated when H2S is passed into acidic solutions of Ge(IV) and Sn(IV) compounds. Some sulfides have cluster structures.
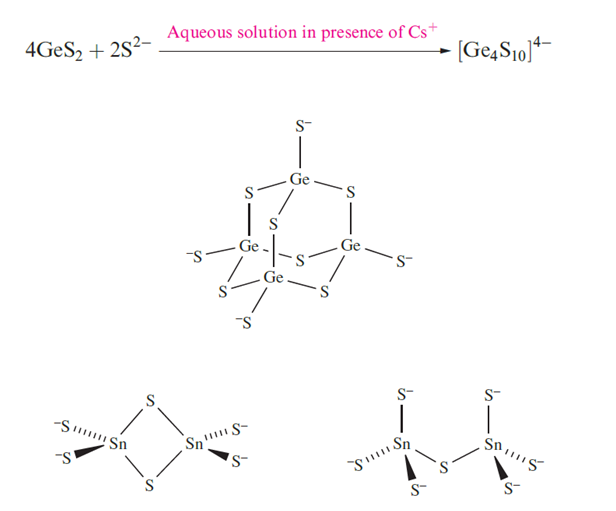
Tin(IV) forms a number of thiostannates containing discrete anions, e.g. Na4SnS4 contains the tetrahedral [SnS4]4- ion, and Na4Sn2S6 and Na6Sn2S7 . The monosulfides of Ge, Sn and Pb are all obtained by precipitation from aqueous media. Both GeS and SnS crystallize with layer structures similar to that of black phosphorus. Lead(II) sulfide occurs naturally as galena and adopts an NaCl lattice. Its formation as a black precipitate (Ksp ≈ 10-30) is observed in the qualitative test for H2S. The colour and very low solubility of PbS suggest that it is not a purely ionic compound.
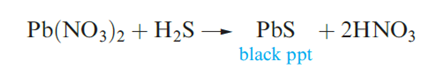
Pure PbS is a p-type semiconductor when S-rich, and an n-type when Pb-rich. It exhibits photoconductivity and has applications in photoconductive cells, transistors and photographic exposure meters. If a material is a photoconductor, it absorbs light with the result that electrons from the valence band are excited into the conducting band; thus, the electrical conductivity increases on exposure to light.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


