
The symmetry elements of objects
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص403-405
الجزء والصفحة:
ص403-405
 2025-11-30
2025-11-30
 175
175
The symmetry elements of objects
Some objects are ‘more symmetrical’ than others. A sphere is more symmetrical than a cube because it looks the same after it has been rotated through any angle about any diameter. A cube looks the same only if it is rotated through certain angles about specific axes, such as 90°, 180°, or 270°about an axis passing through the centres of any of its opposite faces (Fig. 12.1), or by 120°or 240°about an axis passing through any of its opposite corners. Similarly, an NH3molecule is ‘more symmetrical’ than an H2O molecule because NH3 looks the same after rotations of 120°or 240°about the axis shown in Fig. 12.2, whereas H2O looks the same only after a rotation of 180°. An action that leaves an object looking the same after it has been carried out is called asymmetry operation. Typical symmetry operations include rotations, reflections, and inversions. There is a corresponding symmetry element for each symmetry operation, which is the point, line, or plane with respect to which the symmetry operation is performed. For instance, a rotation (a symmetry operation) is carried out around an axis (the corresponding symmetry element). We shall see that we can classify molecules by identifying all their symmetry elements, and grouping together molecules that
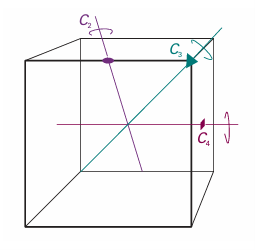
Fig. 12.1 Some of the symmetry elements of a cube. The twofold, threefold, and fourfold axes are labelled with the conventional symbols.
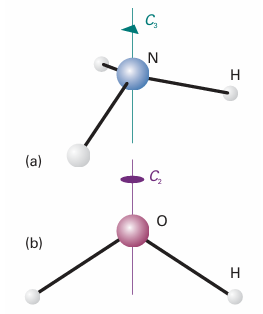
Fig. 12.2 (a) An NH3 molecule has a threefold (C3) axis and (b) an H2O molecule has a twofold (C2) axis. Both have other symmetry elements too.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


