
Partial molar Gibbs energies
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص138-139
الجزء والصفحة:
ص138-139
 2025-11-12
2025-11-12
 216
216
Partial molar Gibbs energies
The concept of a partial molar quantity can be extended to any extensive state function. For a substance in a mixture, the chemical potential is defined as the partial molar Gibbs energy:
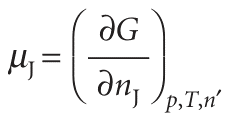
That is, the chemical potential is the slope of a plot of Gibbs energy against the amount of the component J, with the pressure and temperature (and the amounts of the other substances) held constant (Fig. 5.4). For a pure substance we can write G = nJGJ,m, and from eqn 5.4 obtain µJ = GJ,m: in this case, the chemical potential is simply the molar Gibbs energy of the substance, as we used in Chapter 4. By the same argument that led to eqn 5.3, it follows that the total Gibbs energy of a binary mixture is
G=nAµA+nBµB
where µA and µB are the chemical potentials at the composition of the mixture. That is, the chemical potential of a substance in a mixture is the contribution of that substance to the total Gibbs energy of the mixture. Because the chemical potentials depend on composition (and the pressure and temperature), the Gibbs energy of a mixture may change when these variables change, and for a system of components A, B, etc., the equation dG = Vdp − SdT becomes
dG=Vdp−SdT+µAdnA+µBdnB+···
This expression is the fundamental equation of chemical thermodynamics. Its implications and consequences are explored and developed in this and the next two chapters. At constant pressure and temperature, eqn 5.6 simplifies to
dG=µAdnA+µBdnB+ · · ·
We saw in Section 3.5e that under the same conditions dG = dwadd,max. Therefore, at constant temperature and pressure,
dwadd,max = µAdnA + µBdnB + · · ·
That is, additional (non-expansion) work can arise from the changing composition of a system. For instance, in an electrochemical cell, the chemical reaction is arranged to take place in two distinct sites (at the two electrodes). The electrical work the cell per forms can be traced to its changing composition as products are formed from reactants.
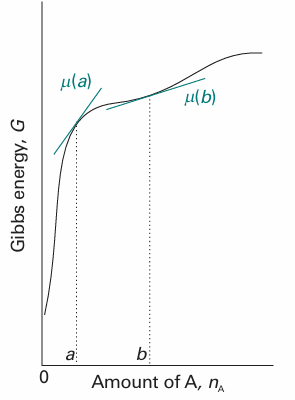
Fig. 5.4 The chemical potential of a substance is the slope of the total Gibbs energy of a mixture with respect to the amount of substance of interest. In general, the chemical potential varies with composition, as shown for the two values at a and b. In this case, both chemical potentials are positive.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


