
Partial molar volume
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص136-138
الجزء والصفحة:
ص136-138
 2025-11-12
2025-11-12
 466
466
Partial molar volume
Imagine a huge volume of pure water at 25°C. When a further 1 mol H2O is added, the volume increases by 18 cm3 and we can report that 18 cm3mol−1is the molar volume of pure water. However, when we add 1 mol H2O to a huge volume of pure ethanol, the volume increases by only 14 cm3. The reason for the different increase in volume is that the volume occupied by a given number of water molecules depends on the identity of the molecules that surround them. In the latter case there is so much ethanol present that each H2O molecule is surrounded by ethanol molecules, and the packing of the molecules results in the H2O molecules increasing the volume by only 14 cm3. The quantity 14 cm3 mol−1 is the partial molar volume of water in pure ethanol. In general, the partial molar volume of a substance A in a mixture is the change in volume per mole of A added to a large volume of the mixture. The partial molar volumes of the components of a mixture vary with composition because the environment of each type of molecule changes as the composition changes from pure A to pure B. It is this changing molecular environment, and the consequential modification of the forces acting between molecules, that results in the variation of the thermodynamic properties of a mixture as its composition is changed. The partial molar volumes of water and ethanol across the full composition range at 25°C are shown in Fig. 5.1. The partial molar volume, VJ, of a substance J at some general composition is defined formally as follows:

where the subscript n′ signifies that the amounts of all other substances present are constant.1 The partial molar volume is the slope of the plot of the total volume as the amount of J is changed, the pressure, temperature, and amount of the other components being constant (Fig. 5.2). Its value depends on the composition, as we saw for water and ethanol. The definition in eqn 5.1 implies that, when the composition of the mixture is changed by the addition of dnA of A and dnB of B, then the total volume of the mixture changes by
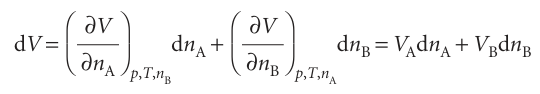
Provided the composition is held constant as the amounts of A and B are increased, the final volume of a mixture can be calculated by integration. Because the partial molar volumes are constant (provided the composition is held constant throughout the integration) we can write
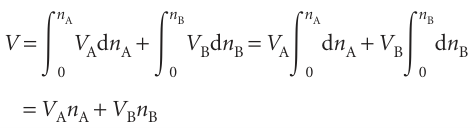
Although we have envisaged the two integrations as being linked (in order to preserve constant composition), because V is a state function the final result in eqn 5.3 is valid however the solution is in fact prepared. Partial molar volumes can be measured in several ways. One method is to measure the dependence of the volume on the composition and to fit the observed volume to a function of the amount of the substance. Once the function has been found, its slope can be determined at any composition of interest by differentiation.
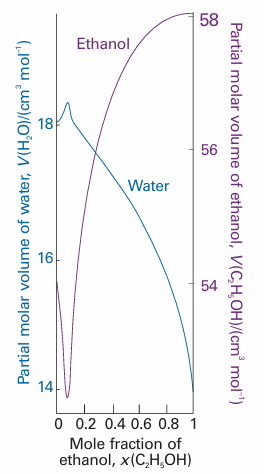
Fig. 5.1 The partial molar volumes of water and ethanol at 25°C. Note the different scales (water on the left, ethanol on the right).
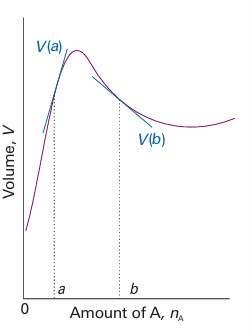
Fig. 5.2 The partial molar volume of a substance is the slope of the variation of the total volume of the sample plotted against the composition. In general, partial molar quantities vary with the composition, as shown by the different slopes at the compositions a and b. Note that the partial molar volume at b is negative: the overall volume of the sample decreases as A is added.

Molar volumes are always positive, but partial molar quantities need not be. For ex ample, the limiting partial molar volume of MgSO4 in water (its partial molar volume in the limit of zero concentration) is −1.4 cm3 mol−1, which means that the addition of 1 mol MgSO4 to a large volume of water results in a decrease in volume of 1.4 cm3. The mixture contracts because the salt breaks up the open structure of water as the ions become hydrated, and it collapses slightly.
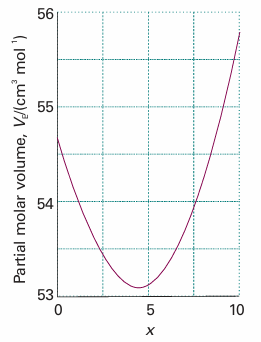
Fig. 5.3 The partial molar volume of ethanol as expressed by the polynomial in Illustration 5.1.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


