

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Stringent Control by Stable RNA Transcription
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
5-6-2021
1830
Stringent Control by Stable RNA Transcription
KEY CONCEPTS
- Poor growth conditions cause bacteria to produce the small-molecule regulators (p)ppGpp.
- The trigger for the reaction is the entry of uncharged tRNA into the ribosomal A site.
- (p)ppGpp competes with ATP during formation of the open complex during transcription initiation by RNA polymerase and inhibits the reaction.
Bacterial rRNA genes are multicopy genes and are dispersed in the genome. E. coli has seven copies of a transcription unit that contains the 16S, 23S, and 5S rRNA genes, in addition to several tRNA genes in the transcribed spacers, as illustrated in FIGURE 1. rRNA and tRNA are stable RNAs that are required to be made only when the cell is growing; the primary level of control of transcription is growth control. As long as E. coli has a sufficient supply of ATP, the cells will continue to divide. Every division requires a doubling of ribosomes, and thus rRNA (as well as tRNA). The primary level of control of transcription of stable RNAs is thus the concentration of ATP.

FIGURE 1. The E. coli rRNA operon structure. The two promoters, the P1 major and the P2 minor promoters, are shown as arrows. Coding regions for 16S, one tRNA, 23S, and 5S are indicated in pink. Transcribed spacers (TS) are shown in green. The two terminators (t) are at the end of the operon.
A second level of control of transcription of stable RNAs exists called stringent response. When bacteria find themselves in such poor growth conditions that they lack a sufficient supply of amino acids to sustain translation, they shut down a wide range of activities. It can be viewed as a mechanism for surviving hard times: The bacterium conserves its resources by engaging in only the minimum of activities and channeling resources into the synthesis of amino acids.
The stringent response causes a massive (10- to 20-fold) reduction in the synthesis of rRNA and tRNA. This alone is sufficient to reduce the total amount of RNA synthesis to 5% to 10% of its previous level. The synthesis of certain mRNAs is reduced, leading to an approximately 33-fold overall reduction in mRNA synthesis. The rate of protein degradation is increased. Many metabolic adjustments occur, as seen in reduced synthesis of nucleotides, carbohydrates, and lipids.
The stringent response is controlled by two unusual nucleotides, ppGpp, guanosine tetraphosphate with diphosphates attached to both the 5′ and 3′ positions, and pppGpp, guanosine pentaphosphate with a 5′ triphosphate and a 3′ diphosphate group, together denoted as (p)ppGpp. These nucleotides are typical small-nucleotide effectors, like the second messenger cAMP , that function by binding to target proteins to alter their activities.
Deprivation of any one amino acid or a mutation that inactivates any aminoacyl-tRNA synthetase is sufficient to initiate the stringent response. The trigger that sets the entire series in motion is the presence of uncharged tRNA in the A site of the ribosome. Under normal conditions only aminoacyl-tRNA is placed in the A site , but when there is not enough aminoacyl-tRNA available to respond to a particular codon the uncharged tRNA becomes able to gain entry.
Bacterial mutants that cannot produce the stringent response are called relaxed mutants. The most common site of relaxed mutation lies in the gene relA, which codes for a protein called the stringent factor. This factor is associated with ribosomes—although the amount is rather low, about 1 molecule for every 200 ribosomes—so probably only a minority of ribosomes is able to produce the stringent response.
The presence of uncharged tRNA in the A site blocks translation, triggering an idling reaction by wild-type ribosomes. Provided that the A site is occupied by an uncharged tRNA specifically responding to the codon, the RelA protein catalyzes a reaction in which ATP donates a pyrophosphate group to the 3′ position of either GTP or GDP.
FIGURE 2 shows the pathway for synthesis of (p)ppGpp. The RelA enzyme uses GTP as substrate more frequently than GDP, so that pppGpp is the predominant product. However, pppGpp is converted to ppGpp by several enzymes. The production of ppGpp via pppGpp is the most common route, and ppGpp is the usual effector of the stringent response. How is ppGpp removed when conditions return to normal? A gene called spoT encodes an enzyme that provides the major catalyst for ppGpp degradation.
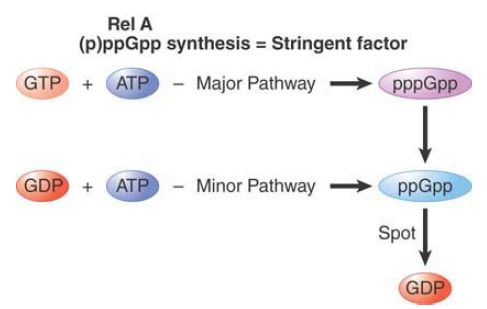
FIGURE 1. Stringent factor catalyzes the synthesis of pppGpp and ppGpp; ribosomal proteins can dephosphorylate pppGpp to ppGpp. ppGpp is degraded when it is no longer needed.
ppGpp is an effector for controlling several reactions, most prominently transcription. It activates transcription at some promoters, such as those involved in amino acid biosynthesis, but its major effect is to inhibit the synthesis of the stable RNA operons —rRNA (and tRNA). The unusual sequence of the major promoter of E. coli’s rRNA genes results in a potentially unstable open complex with RNA polymerase during initiation of transcription and will collapse if the ATP concentration is too low. This class of promoter also requires the activity of a transcription factor, DksA, to bind to RNA polymeraseto effect the strin gent response. ppGpp competes with ATP for the first nucleotide to stimulate this collapse, effectively inhibiting rRNA transcription.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















