


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 25-4-2016
Date: 29-11-2015
Date: 3-5-2021
|
Endocytosis
Endocytosis is the process whereby eukaryotic cells internalize material from their surrounding environment. Internalization is achieved by the formation of membrane-bound vesicles at the cell surface that arise by progressive invagination of the plasma membrane, followed by pinching off) scission), and the release of free vesicles into the cytoplasm (Fig. 1). Several different types of vesicle are generated. Each carries extracellular fluid, macromolecules, and particles into the cell. Extracellular materials are either free within the lumen of the vesicle or bound to its lumenal surface. Endocytosis is believed to be essential for cell viability and is required for numerous cellular functions, including (1) the provision of certain essential nutrients; (2) the removal of unwanted, nonfunctional or potentially harmful material from the extracellular medium; (3) the internalization, processing, and presentation of antigens by major histocompatibility complex (MHC) molecules; (4) the transfer of macromolecules across polarized epithelial cells; and (5) the transmission of neuronal, metabolic, and proliferative signals (see Growth Factors; Insulin). In addition, endocytosis maintains cellular homeostasis by continually monitoring the plasma membrane composition and selectively removing redundant or defective glycoproteins, lipids, or glycolipids for repair or degradation. Endocytosis mediates the rapid recovery of membrane components inserted into the plasma membrane during secretion and is crucial for regenerating of synaptic vesicles. Endocytosis is also used by a range of viral [eg, influenza virus (1)], bacterial (eg, Legionella pneumophila), and protozoan (eg, Toxoplasma gondii) pathogens, and certain protein toxins produced by plants or bacteria (eg, Diphtheria toxin; Cholera toxin), to enter target cells (2).
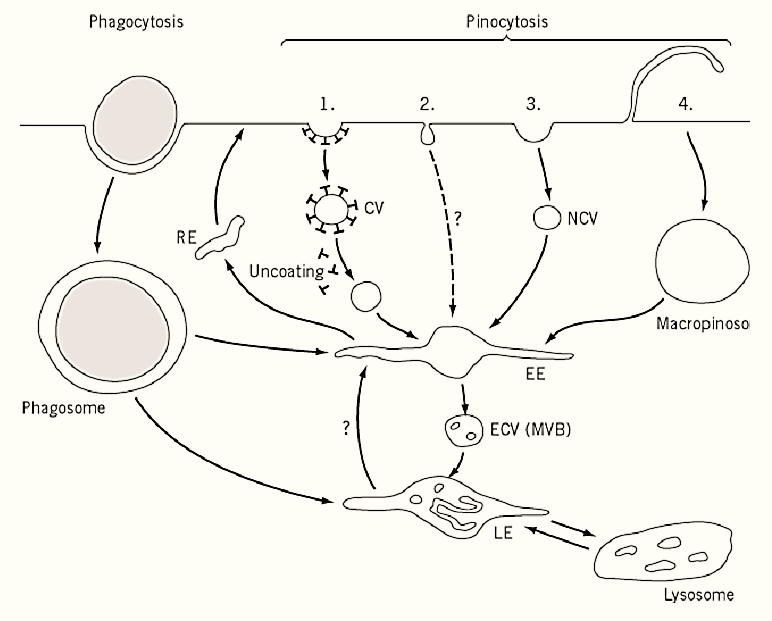
Figure 1. Schematic illustration of endocytic pathways. Four different mechanisms for pinocytosis are illustrated: (1) via clathrin-coated pits and vesicles (CV); (2) via caveolae; (3) via noncoated pits and vesicles (NCV); and (4) via macropinosomes. The abbreviations used are EE, early endosome; LE, late endosome; RE, recycling endosome; ECV, endosomal carrier vesicle; and MVB, multivesicular body.
1. The Endocytic Pathway
The endocytic pathway encompasses all of the organelles in the cell that are involved in endocytosis. The pathway starts at the cell surface, which provides the membrane that forms endocytic vesicles and contains the receptors responsible for binding specific ligands internalized by these vesicles. Endocytic vesicles [phagosomes, clathrin-coated vesicles, caveolae, macropinosomes, and nonclathrin vesicles] derive from the plasma membrane and are directed to fuse with and deliver their membrane and content to the intracellular organelles of the endocytic pathway, the endosomes and lysosomes.
Lysosomes are usually regarded as the graveyard of the cell, the site where ligands internalized from the cell surface and destined for degradation are delivered and hydrolyzed. Although many receptors recycle to the cell surface, others, such as growth factor receptors, remain associated with their ligands and are delivered to lysosomes for degradation. Lysosomes are usually vesicular (~0.5 to 1 µm in diameter) and are located primarily, although not exclusively, in the perinuclear region of the cell. The pH within lysosomes can be as low as 4.8, the optimum for the many hydrolytic enzymes they contain. They derive their content from late endosomes, probably through repeated cycles of fusion with and fission from these organelles (3). In antigen-presenting cells, late endosomes and lysosomes contain large amounts of MHC II antigens and have been termed MIICs ( (4. In addition, lysosomes in haematopoietic cells are secretory organelles that undergo regulated fusion with the plasma membrane (5).
The membrane proteins, lipids, and hydrolases that make up the membrane and content of endocytic organelles are synthesized in the endoplasmic reticulum (ER) and Golgi apparatus and are subsequently transported to endosomes and lysosomes by vesicular transport. Many hydrolases carry the mannose 6-phosphate recognition marker that enables delivery via the mannose 6-phosphate receptors. By contrast, the membrane proteins of endosomes and lysosomes carry sorting signals that facilitate their delivery.
The endocytic pathway is extremely dynamic. In some tissue culture cells, approximately 1500 coated vesicles internalize from the cell surface every minute. This is equivalent to internalizing the membrane of the entire cell surface every 30 to 60 min (6). The surface area of early endosomes is approximately one-fifth that of the cell surface, and the entire surface area of these organelles turns over approximately every 12 min (7). Receptors, such as the low-density lipoprotein (LDL( receptor, are internalized very rapidly at rates of 10 to 30% of the cell surface pool per min and are recycled in 10 to 15 min back to the cell surface. Each individual receptor may mediate many rounds of internalization and recycling in its life time (8). The organization of endocytic organelles within the cell is regulated by interaction with cytoskeletal elements. Actin-based systems have been implicated in the formation of endocytic vesicles from the plasma membrane, and actin/myosin and the microtubule system have been implicated in moving and positioning endocytic organelles within the cell.
2. Endocytic Mechanisms
Classically, endocytosis has been divided into phagocytosis (“cellular eating”) and (pinocytosis “cellular drinking”) (Fig. 1). Phagocytosis describes the internalization of large particles (>0.2 µM in diameter) following particle binding to specific plasma membrane receptors. Therefore, phagocytosis is receptor-mediated and depends on the presence of appropriate ligands. Pinocytosis is the formation of generally smaller vesicles (50 to 150 nm diameter) that transport extracellular fluid )fluid-phase endocytosis) and macromolecules specifically (receptor-mediated endocytosis) or nonspecifically (adsorptive endocytosis) bound to the plasma membrane. These vesicles are often formed constitutively from clathrin-coated pits independently of the presence of specific ligands, but there are alternatives to clathrin-mediated uptake. The different endocytic mechanisms, more than one of which coexist and function in a single cell, are classified as phagocytosis, pinocytosis, macropinocytosis, and noncoated vesicles.
2.1. Noncoated vesicles
Endocytosis still occurs when clathrin-mediated uptake is blocked, as evidenced by the internalization of ricin, interleukin-2, and fluid-phase markers. Morphologically, the vesicles thought to mediate this uptake noncoated, ~80 nm in diameter, and distinct from caveolae and macropinosomes. At present, nothing is known about the molecular mechanisms responsible for this uptake process, although the continued uptake of ricin in the presence of a dominant-negative mutant form of dynamin suggests that it may be dynamin-independent (9. (
3. Endocytosis Signals
Receptors and other proteins that undergo efficient endocytosis from the cell surface contain endocytosis signals. The signals associated with proteins that internalize through clathrin-coated pits are those that are best characterized. Four classes of endocytosis signal have been identified and studied in some detail. First are tyrosine residues within motifs similar to Phe-X-Asn-Pro-X-Tyr or Tyr-X-X-O (where X = any amino acid and O = a large hydrophobic amino acid), which are the endocytosis motifs identified in the LDL receptor and transferrin receptor, respectively (10). It has been proposed that these motifs form turn structures and interact either directly with clathrin (11) or with the m2 subunit of the AP-2 complex [(10) see Pinocytosis], respectively. Generally these signals are constitutively active, and proteins that contain them undergo continuous endocytosis and recycling.
A second group of signals requires pairs of hydrophobic amino acids, frequently leucine residues, although isoleucine, methionine, or valine substitutes for one of the leucines in some circumstances [e.g., MHC class II invariant chain (12)]. These so-called dileucine signals are less well characterized. There is evidence, however, that they also bind AP-2 complexes through a site or sites distinct from those bound by the Tyr-based signals. There is also evidence that in some cases (e.g., CD4) the activity of these signals is regulated by phosphorylation of adjacent serine residues (13). The signal is active when the motif is phosphorylated and inactive when dephosphorylated.
The third class of internalization signal has been identified in members of the family of seven transmembrane-domain heterotrimeric G protein-coupled receptors that are internalized following ligand binding. For several of these receptors, in particular the b2-adrenergic receptor, ligand-induced phosphorylation of serine residues in the serine-rich C-terminal domain of the molecule leads to recruitment of b-arrestins that uncouple associated heterotrimeric G proteins and function as adaptor complexes to recruit the receptor into clathrin-coated pits (14). Finally, in S. cerevisiae, a factor-induced phosphorylation of serine residues in the Ste2p receptor protein C-terminal domain leads to ubiquitination of Ste2p and its subsequent internalization (15). A number of mammalian cell surface receptors, including the growth hormone receptor, are also ubiquitinated following ligand binding (16). Whether this modification is required for endocytosis of the receptor and how the modification facilitates interaction with the endocytosis machinery are unclear.
Sorting signals also operate within endosomes and other sites in the endocytic pathway to specify the routing of specific proteins. The signals themselves are not well characterized. Recycling to the plasma membrane is the default pathway and may not require specific signals. However, the Tyr-X-X-O and dileucine motifs discussed previously function to target internalized proteins from early endosomes to lysosomes (12), and receptor cross-linking by multivalent ligands or antibody complexes directs sorting from early endosomes to lysosomes (17). The low pH of endocytic compartments may also influence sorting by inducing dissociation of pH-sensitive ligand-receptor interactions. Other signals, for example, a diaromatic amino acid-containing motif in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor, function as a lysosome-avoidance motif to prevent proteins from being transported to lysosomes. How these sorting signals are interpreted is unclear for the most part, although coat proteins and adaptors have been implicated in certain steps (19). A subset of the COP1 coatomer complex has been found associated with early endosomes (20), as have clathrin and AP adaptor complexes.
4. Medical Significance
The endocytic pathway is crucial for many cellular functions. Defects in functions associated with the endocytic pathway have been linked to a number of clinical conditions. For example, failure to internalize serum LDL is associated with atherosclerosis, and defects in producing or effectively targeting lysosomal hydrolases results in a range of inherited lysosomal storage diseases. It is believed that the problems associated with Chediak-Higashi syndrome result from defects in sorting associated with late endosomes and lysosomes. Chromosomal translocations of genes that encode proteins involved in coated vesicle formation have been found in a number of lymphomas and leukemias. In addition, many bacterial, protozoan, and viral pathogens enter cells and establish infection through the endocytic pathway. It is likely that many other conditions will be linked to endocytic functions. However, the endocytic pathway is likely to provide one of the most effective routes to target and deliver drugs, DNA, or other therapeutic agents to cells.
References
1. M. Marsh and A. Helenius (1989) Adv. Virus Res. 36, 107–151.
2. J. M. Lord and L. M. Roberts (1998) J. Cell Biol. 140, 733–736.
3. B. M. Mullock, N. A. Bright, C. W. Fearon, S. R. Gray, and J. P. Luzio (1998) J. Cell Biol. 140, 591-601.
4. I. Mellman, P. Pierre, and S. Amigorena (1995) Curr. Opinion Cell Biol. 7, 564–572.
5. G. M. Griffiths (1997) Semin. Immunol. 9, 109–15.
6. M. Marsh and A. Helenius (1980) J. Mol. Biol. 142, 439–454.
7. G. Griffiths, R. Back, and M. Marsh (1989) J. Cell Biol. 109, 2703–2720.
8. J. L. Goldstein, M. S. Brown, R. G .W. Anderson, and D. W. Russell (1985) Annu. Rev. Cell Biol. 1, 1–39.
9. C. Lamaze and S. L. Schmid, (1995) Curr. Opinion Cell Biol. 7, 573–580.
10. T. Kirchhausen, J. S. Bonifacino, and H. Riezman (1997) Curr. Opinion Cell Biol. 9, 488–495.
11. R. G. Kibbey, J. Rizo, L. M. Gierasch, and R. G. W. Anderson (1998) J. Cell Biol. 142, 59–67.
12. I. Sandoval and O. Bakke (1994) Trends Cell Biol. 4, 292–297.
13. M. Marsh and A. Pelchen-Matthews (1996) In Current Topics in Microbiology and Immunology, Vol. 205 (D. R. Littman, ed.), Springer pp. 107–135.
14. S. S. Ferguson, L. S. Barak, J. Zhang, and M. G. Caron (1996) Can. J. Physiol. Pharmacol. 741095, -1110.
15. L. Hicke and H. Riezman (1996) Cell 84, 277–287.
16. R. Govers, P. van Kerkhof, A. L. Schwartz, and G. J. Strous (1997) EMBO J. 16, 4851–4858.
17. P. Ukkonen, V. Lewis, M. Marsh, A. Helenius, and I. Mellman (1986) J. Exp. Med. 163, 952–971.
18. A. Schweizer, S. Kornfeld, and J. Rohrer (1997) Proc. Natl. Acad. Sci. USA 94, 14471–14476.
19. E. Diaz and S. R. Pfeffer (1998) Cell 93, 433–443.
20. F. Aniento, F. Gu, R. G. Parton, and J. Gruenberg (1996) J. Cell Biol. 133, 29–41.



|
|
|
|
صنع الذكريات والتفكير يدمر الدماغ.. دراسة تشرح السبب
|
|
|
|
|
|
|
الصين.. عودة كاسحتي الجليد إلى شنغهاي بعد انتهاء بعثة استكشافية إلى القطب الجنوبي
|
|
|
|
|
|
جامعة الكفيل تكرم الفائزين بأبحاث طلبة كلية الصيدلة وطب الأسنان
|
|
|
|
مشروع التكليف الشرعي بنسخته السادسة الورود الفاطمية... أضخم حفل لفتيات كربلاء
|
|
|
|
ضمن جناح جمعيّة العميد العلميّة والفكريّة المجمع العلمي يعرض إصداراته في معرض تونس الدولي للكتاب
|
|
|
|
جامعة الكفيل تعقد مؤتمرها الطلابي العلمي الرابع
|