


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-5-2017
Date: 2-10-2020
Date: 1-7-2019
|
Diazomethane, Carbenes, and Cyclopropane Synthesis
A carbenoid is best considered to be a reagent which, while not actually a carbene, behaves as if it were an intermediate of this type.
Dichlorocarbenes can also form cyclopropane structures and are created in situ from reagents such as chloroform and KOH.
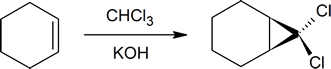
The detailed mechanism of the formation of dichlorocarbene is given below. Note that the deprotonation of chloroform generates the trichloromethanide anion, which spontaneously expels the chloride anion.
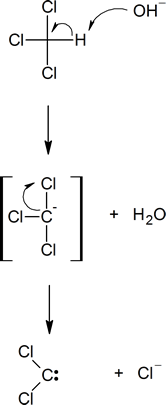
The highly strained nature of cyclopropane compounds makes them very reactive and interesting synthetic targets. Additionally cyclopropanes are present in numerous biological compounds. One common method of cyclopropane synthesis is the reaction of carbenes with the double bond in alkenes or cycloalkenes. Methylene, H2C, is simplest carbene, and in general carbenes have the formula R2C. Other species that will also react with alkenes to form cyclopropanes but do not follow the formula of carbenes are referred to as carbenoids.
Carbenes were once only thought of as short lived intermediates. The reactions of this section only deal with these short lived carbenes which are mostly prepared in situ, in conjunction with the main reaction. However, there do exist so called persistent carbenes. These persistent carbenes are stabilized by a variety of methods often including aromatic rings or transition metals. In general a carbene is neutral and has 6 valence electrons, 2 of which are non bonding. These electrons can either occupy the same sp2 hybridized orbital to form a singlet carbene (with paired electrons), or two different sp2 orbitals to from a triplet carbene (with unpaired electrons). The chemistry of triplet and singlet carbenes is quite different but can be oversimplified to the statement: singlet carbenes usually retain stereochemistry while triplet carbenes do not. The carbenes discussed in this section are singlet and thus retain stereochemistry.
The reactivity of a singlet carbene is concerted and similar to that of electrophilic or nucleophilic addition (although, triplet carbenes react like biradicals, explaining why sterochemistry is not retained). The highly reactive nature of carbenes leads to very fast reactions in which the rate determining step is generally carbene formation.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
أصواتٌ قرآنية واعدة .. أكثر من 80 برعماً يشارك في المحفل القرآني الرمضاني بالصحن الحيدري الشريف
|
|
|